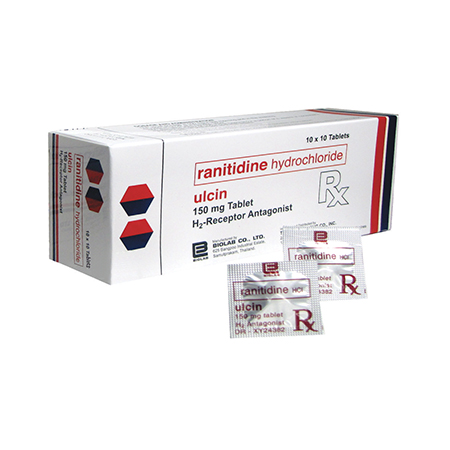
Description
DESCRIPTION
FORMULATION
Each tablet contains:
Ranitidine (as hydrochloride) …………………… 150 mg
PHARMACOLOGY
Ranitidine (ULCIN Injection) is a specific, rapidly acting histamine H2 – receptor antagonist. It inhibits basal and stimulated secretion of gastric acid, reducing both volume of the acid and pepsin content of the secretion.
PHARMACOKINETICS
Ranitidine is rapidly absorbed from the gastrointestinal tract with peak plasma concentration occurring about 2 to 3 hours after administration by mouth. Food does not significantly impair absorption. The bioavailability of ranitidine following oral administration is about 50%. It is weakly bound, about 15%, to plasma proteins. The elimination half-life is about 2 to 3 hours and suppresses gastric acid secretion for about 12 hours. A small portion of ranitidine is metabolized in the liver to the N-oxide, S-oxide, and desmethylranitidine; the N-oxide is the major metabolite but accounts for only about 4 to 6% of a dose. Approximately 30% of an oral dose and 70% of an intravenous dose is excreted unchanged in the urine in 24 hours, primarily by active tubular secretion; there is some excretion in the feces. Ranitidine crosses placental barrier and is distributed into breast milk.
INDICATIONS
Ranitidine (ULCIN Injection) is indicated for all conditions where a controlled reduction of acid secretion of the stomach is required. Such conditions include duodenal ulcer, gastric ulcer, esophagitis, and Zollinger-Ellison Syndrome.
DOSAGE AND ADMINISTRATION
The usual dose is 150 mg in the morning and in the evening.
Duodenal ulcer, gastric ulcer: the recommended dosage is 150 mg twice a day or 300 mg at bedtime for a period of 4 weeks. If necessary, it can be prolonged up to 6-8 weeks.
Esophagitis: the recommended dosage is 150 mg twice a day for a period of 6 weeks. To prevent recurrence the treatment can be continued with a dosage of 150 mg in the evening.
Zollinger-Ellison Syndrome: the initial dosage is 150 mg three times a day and is to be increased if necessary until up to 600 mg – 900 mg.
CONTRAINDICATIONS
RANITIDINE is contraindicated in patients known to have hypersensitivity to the drug.
PRECAUTIONS
Treatment with a histamine H2-receptor antagonist may mask the symptoms associated with carcinoma of the stomach and therefore may delay diagnosis of the condition.
In severe renal impairment, plasma levels of ranitidine are increased. Accordingly, it is recommended in such patients that Ranitidine (ULCIN Injection) be administered in doses of 25 mg.
ADVERSE EFFECTS
Transient and reversible changes in liver function tests can occur.
Diarrhea, muscle pain, dizziness and skin rashes may occasionally occur.
Reversible blood count changes (leucopenia and thrombocytopenia) have occurred in a few patients. Rare cases of agranulocytosis or of pancytopenia, sometimes with marrow hyperplasia, have been reported.
Hypersensitivity reactions (urticaria, angioneurotic adema, bronchospasm, hypotension) have been reported rarely.
Bradycardia, headache, sometimes severe and dizziness habe been reported in a very small proportion of patients.
DRUG INTERACTIONS
RANITIDINE does not inhibit the hepatic cytochrome P450 linked mixed function oxygenase enzyme system. Accordingly, RANITIDINE does not potentiate the action of drugs which are oxidized or inactivated by this enzyme. These include diazepam, lignocaine, phenytoin, propranolol, theophylline and warfarin.
PREGNANCY AND LACTATION
RANITIDINE crosses the placenta but therapeutic doses administered to obstetric patients in labor or undergoing caesarian section have been without any adverse effect on labor, delivery or subsequent neonatal progress.
RANITIDINE is also excreted in human breast milk, should be used during pregnancy only if considered essential.
AVAILABILITY
ULCIN tablets each tablet containing 150 mg Ranitidine is available in boxes of 10 x 10 strip-sealed tablets and 3 x 10 strip-sealed tablets.
STORAGE
Store at temperatures not exceeding 30°C.

Reviews
There are no reviews yet.