Description
The Cathay Drug Co., Inc.
FORMULATION
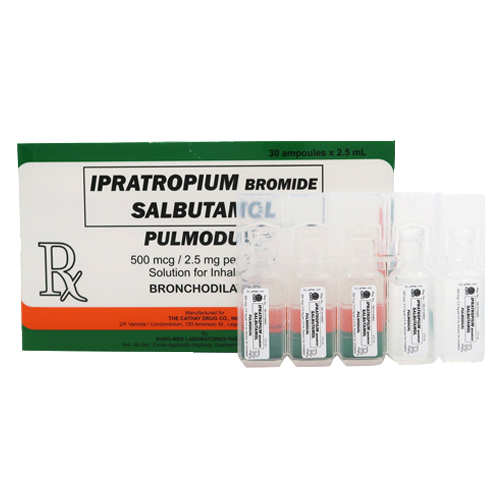
Each ampoule (2.5 mL) contains:
Ipratropium bromide (as monohydrate) ………………………………… 500 mg
Salbutamol (as sulphate) ………………………………………………… 2.5 mg
PHARMACOLOGY
Ipratropium bromide is a synthetic quaternary anticholinergic (parasympathetic) ammonium compound chemically related to atropine. It appears to inhibit vagally mediated reflexes by antagonizing the action of acetylcholine. Anticholinergics prevent the increases in intracellular concentrations of cyclic guanosine monophosphate (cyclic GMP) which are caused by interaction of acetylcholine with the muscarinic receptor on bronchial smooth muscle.
The bronchodilation following inhalation is primarily a local, site – specific effect. Salbutamol sulphate is a direct-acting sympathomimetic with mainly beta-adrenergic activity and a selective action on beta2-receptors (a beta2-agonist) used to produce bronchodilation. It relieves reversible bronchospasm by relaxing the smooth muscles of the bronchioles in conditions associated with asthma, bronchitis, emphysema or bronchiectasis.
Mechanism of Action
Ipratropium bromide
Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released at the neuromuscular junctions in the lung. Anticholinergics prevent the increases in intracellular concentration of Ca++ which is caused by interaction of acetylcholine with the muscarinic receptors on bronchial smooth muscle.
Salbutamol sulfate
Activation of beta2-adrenergic receptors on airway smooth muscle leads to the activation of adenylyl cyclase and to an increase in the intracellular concentration of cyclic-3′,5′-adenosine monophosphate (cyclic AMP). This increase of cyclic AMP leads to the activation of protein kinase A, which inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in relaxation. Salbutamol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Salbutamol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway.
Salbutamol has been shown in most clinical trials to have more bronchial smooth muscle relaxation effect than isoproterenol at comparable doses while producing fewer cardiovascular effects. However, all beta-adrenergic drugs, including albuterol sulfate, can produce a significant cardiovascular effect in some patients.
PHARMACOKINETICS
After inhalation, only a small amount of ipratropium reaches the systemic circulation from the nasal mucosa. Less than 20% of an 84 mcg per nostril dose is absorbed from the nasal mucosa, but the amount which is systemically absorbed from nasal administration exceeds the amount absorbed from inhalation solution (2% of a 500 mcg dose). Some ipratropium is advertently swallowed but it is poorly absorbed from the gastrointestinal tract. Ipratropium is minimally bound (< 9% in vitro) to plasma albumin and α1- acid glycoprotein. It is partially metabolized to inactive ester hydrolysis products. The elimination half-life is about 1.6 hours. Ipratropium and its metabolites are eliminated in the urine and feces.
Salbutamol is readily absorbed from the gastrointestinal tract. It is subject to first-pass metabolism in the liver and possibly in the gut wall; the main metabolite is an inactive sulfate conjugate. Salbutamol sulphate is rapidly excreted in the urine as metabolites and unchanged drug. There is some excretion in the feces. Salbutamol does not appear to be metabolized in the lung, therefore its ultimate metabolism and excretion after inhalation depends upon the delivery method used, which determines the proportion of inhaled salbutamol relative to the proportion inadvertently swallowed. It has been suggested that most of an inhaled dose is swallowed and absorbed from the gut. The plasma half-life of salbutamol is 4 to 6 hours.
INDICATIONS
Ipratropium bromide + Salbutamol (Pulmodual) is indicated in the treatment of reversible airways obstruction, like: asthma and chronic obstructive pulmonary disease for patients in need of more than a single bronchodilator or who require a second bronchodilator.
DOSAGE AND ADMINISTRATION
Per 2.5 mL of Ipratropium bromide + Salbutamol contains 500 mcg of Ipratropium bromide and 2.5 mg of Salbutamol.
Dosage for Solution of Inhalation
Adult (including Elderly Patients) and Adolescents over 12 years:
One (1) ampule (2.5 mL) every 6 to 8 hours.
For the treatment of acute attacks:
One (1) ampule (2.5 mL) is sufficient for the prompt relief of symptom. In severe cases Two (2) ampules (5 mL) may be required if an attack has not been relieved by One (1) ampule (2.5 mL).
Children 2 to 12 years:
3 drops/kg/dose (maximum dose 2.5 mg of salbutamol) every 6 to 8 hours.
Administration
Administer the oral inhalation via nebulization:
- Prepare the nebulizer for use.
- Remove the ampule from the labelled strip by twisting and pulling. Hold the ampule upright and twist off the cap. Transfer the contents of the solution in the nebulizer reservoir.
- Use the nebulizer according to the instruction provided by the manufacturer
- After use, discard any solution left in the reservoir and thoroughly clean the nebulizer.
Since the Ipratropium bromide +Salbutamol (Pulmodual) solution contains no preservatives, it is important to use the content immediately after opening. A new ampule should be used for each administration to avoid microbial contamination. Discard partly used, opened or damaged ampule.
Ipratropium bromide +Salbutamol (Pulmodual) solution for inhalation should not be mixed with other drugs in the same nebulizer.
CONTRAINDICATIONS
Known hypersensitivity to the drug or any other component of Ipratropium bromide +Salbutamol (Pulmodual) or to atropine or its derivatives. Hypertropic obstructive cardiomyopathy or tachyarrhythmia.
WARNINGS AND PRECAUTIONS
Ipratropium bromide +Salbutamol (Pulmodual) should be used with caution in patients with prostatic hypertrophy bladder neck obstruction, narrow angle glaucoma; risk of paradoxical bronchospasm usually occurs with the first dose of nebulized solution. It should be inhaled under medical supervision; hyperthyroidism, cardiovascular disease, arrhythmias, susceptibility to QT-interval prolongation, hypertension and diabetes mellitus.
Potentially serious hypokalemia may result from beta2 agonist therapy, possibly through intracellular shunting, which can produce adverse cardiovascular effects. The decrease in serum potassium levels is usually transient, not requiring supplementation.
SPECIAL POPULATION
Pregnancy
Teratogenic effects: Pregnancy category C
Ipratropium bromide +Salbutamol (Pulmodual) safety during pregnancy (human) has not been established. However, the use of drugs during pregnancy should be observed only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
The use of Ipratropium bromide +Salbutamol (Pulmodual) for the treatment of COPD during labor should be restricted to those patients in whom the benefit clearly outweigh the risk because of the potential for beta-agonist interference with uterine contractility.
Nursing Mothers
The components of Ipratropium bromide +Salbutamol are excreted in human milk. Although lipid-soluble quaternary bases pass into breast milk, it is unlikely that ipratropium bromide would reach the infant to an important extent, especially when taken by inhalation as a nebulized solution. However, caution should be exercised when administering to a nursing mother.
ADVERSE EFFECTS
The side effects of Ipratropium bromide +Salbutamol (Pulmodual) includes: dry mouth, nausea, constipation, headache, fine tremor (particularly in the hands), nervous tension, muscle cramps and palpitation.
Other adverse effects include: tachycardia, atrial fibrillation, arrhythmias, peripheral vasodilation, and disturbances of sleep and behaviour.
Paradoxical bronchospasm, urticaria, angioedema, hypotension, and collapse have also been reported.
Potentially serious hypokalemia may occur from beta2-agonist therapy.
SYMPTOMS AND TREATMENT OF OVERDOSAGE
Reports of overdosage are more likely with Salbutamol. The expected symptoms of overdosage are tachycardia, CNS stimulation, tremor, hypokalemia and hyperglycemia.
Symptomatic treatment of the adverse effects has proved successful. The plasma-potassium concentration and pulse rate have been found to correlate with the plasma concentrations of salbutamol.
DRUG INTERACTIONS
- Anticholinergic agents: Co – administration of other anticholinergic containing drugs must be avoided. It can cause an additive interaction upon concomitant use of anticholinergic medications.
- Beta-adrenergic agents: Caution is advised in the co-administration of Ipratropium bromide +Salbutamol and other sympathomimetic agents due to the increased risk of adverse cardiovascular agents.
- Beta-receptor blocking agents: Beta-receptor blocking agents and salbutamol inhibit the effect of each other, it should be used with caution in patients with hyper reactive airways
- Cardiac glycosides: salbutamol possibly reduces plasma concentration of digoxin
- Diuretics: increased risk of hypokalemia when high doses of beta2 sympathomimetics is given with diuretics (e.g. loop or thiazide diuretics)
- Monoamine oxidase inhibitors or tricyclic antidepressants: it should be administered with extreme caution to patients treated with monoamine oxidase inhibitors or tricyclic antidepressants or within two weeks of discontinuation of such agents because the action of salbutamol sulphate on the cardiovascular system may be potentiated.
- Theophylline: increased risk of hypokalemia when high doses of beta2 sympathomimetics is given with theophylline.
STORAGE
Store at temperatures not exceeding 30°C.
Protect from light. Place inside aluminum pouch.
DISCARD UNUSED AMPULES THREE (3) MONTHS AFTER OPENING FOIL POUCH
NOT FOR INTRAVENOUS USE
FOR ORAL INHALATION ONLY
AVAILABILITY
Ipratropium bromide +Salbutamol (Pulmodual) 500 mcg/2.5 mg per 2.5 mL Solution for Inhalation: 2.5 mL ampule (Box of 30’s)
Manufactured by:
Euro-Med Laboratories Phil., Inc.
Km. 36 Gen. Emilio Aguinaldo Highway, Dasmariñas, Cavite, Philippines
Manufactured for:
The Cathay Drug Co., Inc.
2/F Vernida I Condominium, 120 Amorsolo St. Legaspi Village, Makati City
Date of revision: July 2019

Reviews
There are no reviews yet.