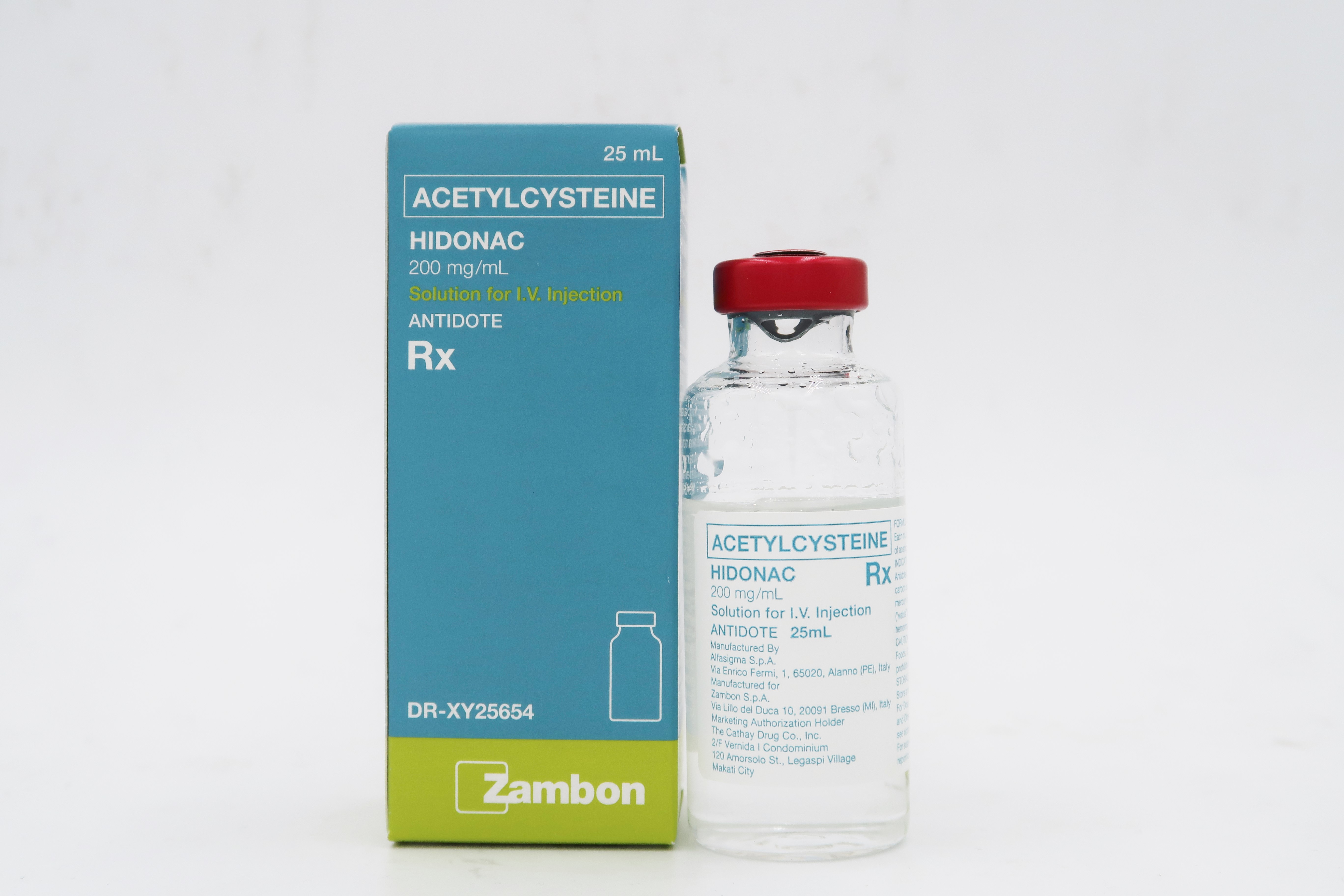
Description
The Cathay Drug Co., Inc.
FORMULATION
Each mL of solution contains 200 mg acetylcysteine, Ph. Eur.
PRODUCT DESCRIPTION
Acetylcysteine (HIDONAC) is a clear, colorless solution with a slightly sulphureous odor. The color may turn from essentially colorless to a very slightly pink or purple upon opening of the vial of once the rubber stopper is punctured. The color change does not affect the quality of the product, it is due to the oxidation of any traces of iron present to the ferric state and subsequent formation of the colored complex with acetylcysteine.
PHARMACOLOGICAL AND PHARMACOKINETIC PROPERTIES
PHARMACOLOGY
N-acetylcysteine (NAC), active ingredient of HIDONAC, is a cysteine derivative provided with a free thiol (-SH) nucleophilic group which is able to interact directly with the electrophilic group of the oxidant radicals. Due to its molecular structure, NAC can easily cross the cellular membranes. Inside the cell, NAC is deacetylated to L-cysteine, an amino acid required for the synthesis of glutathione (GSH). GSH is a highly reactive tripeptide, found ubiquitously in various tissues of animals and it is essential for the maintenance of functional capacity as well as cellular morphological integrity, since it represents the most important protective endocellular mechanism against oxidant radical, either exogenous or endogenous, as well as towards numerous cytotoxic substances.
Due to its antioxidant property and its effectiveness in promoting the synthesis of glutathione, NAC is used as a substrate contributing to the cellular protection from harmful agent which through progressive GSH depletion, would be able to express their cytotoxic action as in the case of paracetamol poisoning that may lead to hepatic failure, encephalopathy and death. NAC administration is effective in the prevention of liver damage maintaining an adequate GSH level. The efficacy of the treatment is most likely to occur when it started early.
TOXICOLOGY
NAC has been evaluated to have low toxicity. After intravenous administration, the LD50 is 2.8 g/kg in rat and 4.5 g/kg in mouse. After oral administration, the LD50 is more than 10 g/kg both in rat and mouse. During the repeated administration studies, the dose of 1 g/kg/die by oral route for 12 weeks has been well tolerated by rats. In dogs, no toxic reactions have been observed after oral administration of 300 mg/kg/die for one year. No teratogenic effect was observed in the offsprings of pregnant rats and rabbits when treated with high dose of NAC during the organogenesis period.
PHARMACOKINETICS
The tmax was approximately 1 hour after oral administration. The bioavailability by the oral route was less than 10% of the dose administered. This finding does not mean that the drug is poorly absorbed but that extensive first-pass metabolism of the drug occurs in the liver. In plasma, NAC s found as N,N-diacetylcysteine and mixed disulfides with other thiols, or of low molecular weight or with proteins. The elimination half-life was 5-6 hours after intravenous administration.
NAC is mainly excreted in the urine. The main urinary metabolite of NAC is the inorganic sulfate. However, in acute paracetamol intoxication, the urinary metabolite of NAC may differ.
INDICATIONS
Used as an antidote in poisoning caused by paracetamol, carbon tetrachloride, arsenic, metallic mercury inhalation and yellow phosphorus (“watusi”) and cyclophosphamide-induced hemorrhagic cystitis.
DOSAGE AND ADMINNISTRATION
For the therapeutic success of the antidote treatment of intoxication with paracetamol, the period between intake of an overdose paracetamol and the start of the therapy is essential. Therapy should be initiated within 0 to 8 hours of paracetamol ingestion. For an administration of acetylcysteine during 15 hours after an overdose of paracetamol, the therapy is mostly ineffective, but there is evidence in literature of a successful treatment 6-24 hours after the intake of paracetamol.
The injection is administered by intravenous infusion. The infusion must be carried out slowly in order to reduce the risk of undesirable effects.
Acetylcysteine (HIDONAC) should be administered by intravenous infusion preferably using Glucose 5% as the infusion fluid. Sodium Chloride 0.9% solution may be used if Glucose 5% is not suitable.
Adults
The full course of treatment with acetylcysteine (HIDONAC) comprises 3 consecutive intravenous infusion:
- First Infusion
- Initial loading dose of 150 mg/kg body weight in 200 mL over 1 hour
- Second infusion
- 50 mg/kg in 500 mL over the next 4 hours.
- Third infusion
- 100 mg/kg in 1 L over the next 16 hours.
The patient should therefore receive a total of 300 mg/kg over a 21 hour period.
A ceiling weight of 110 kg should be used when calculating the dosage for obese patients. Dosage should be calculated using the patient’s actual weight.
Children
Children should be treated with the same doses and regimen as adults; however, the quantity of intravenous fluid must be modifies to take into account age and weight, as fluid overload is a potential danger.
N-acetylcyeteine (HIDONAC) should be administered by intravenous infusion preferably using Glucose 5% as the infusion fluid. Sodium Chloride 0.9% solution may be used if Glucose 5% is not suitable.
Doses should be administered using an appropriate infusion pump.
The full course of treatment with acetylcysteine (HIDONAC) comprises 3 consecutive intravenous infusion:
- First Infusion
- Initial loading dose of 150 mg/kg body weight infused over 1 hour (150 mg/kg/h). Given as a 50 mg/mL solution at a rate of 3 mL/kg/h.
- Second infusion
- 50 mg/kg infused over 4 hours (12.5 mg/kg/h). Given as a 6.25 mg/mL solution at a rate of 2 mL/kg/h.
- Third infusion
- 100 mg/kg infused over 16 hours (6.25 mg/kg/h). Given as a 6.25 mg/mL solution at a rate of 1 mL/kg/h.
Preparation of the solution
Dose 1
Prepare a 50 mg/mL solution. Dilute 10 mL of N-acetylcysteine (200 mg/mL) with 30 mL glucose 5% or sodium chloride 0.9% to give a total volume of 40 mL.
Dose 2
Prepare a 6.25 mg/mL solution. Dilute 10 mL of N-acetylcysteine (200 mg/mL) with 310 mL glucose 5% or sodium chloride 0.9% to give a total volume of 3200mL.
Dose 3
Prepare a 6.25 mg/mL solution. Dilute 10 mL of N-acetylcysteine (200 mg/mL) with 310 mL glucose 5% or sodium chloride 0.9% to give a total volume of 320 mL.
Administration
Weigh the child.
Determine the total volume of solution to be infused (infusion fluid + N-acetylcysteine prepared as above) into the child from the table. A separate volume will be required for each of the three infusion periods
CONTRAINDICATIONS
Known hypersensitivity to the drug or to other chemically related substances.
INSTRUCTIONS FOR USE AND HANDLING
Acetylcysteine (HIDONAC) is a sterile solution and must be diluted according to the scheme described under “Dosage and Administration”, taking into consideration the volume of infusion and weight of the patient.
The solution must be administered through intravenous infusion after dilution. The diluted solution is stable for 24 hours when stored at 25° C.
WARNINGS AND PRECAUTIONS
Anaphylactic reactions may occur when intravenous acetylcysteine is directly administered at high doses as well as when a rapid rate is used. Treatment of such reactions must be symptomatic. Patients suffering from bronchial asthma or with a history of asthma must be closely monitored since bronchospasm may occur during treatment. In such event, acetylcysteine must be suspended immediately and appropriate treatment must be initiated.
It should be used with caution in asthmatic patients and patients with a history of peptic ulceration.
Caution should be used in the administration of antidotal doses in patients with bodyweight less than 40 kg because of the possible risk of fluid overload with subsequent hyponatremia, seizure, and death. It is therefore recommended to strictly follow the indications reported under “Dosage and Administration”.
This medicinal product contains 748 mg sodium per vial (32.5 mmol). To be taken into consideration by patients on a controlled sodium diet.
Acetylcysteine should be given by intravenous route under strict medical supervision. The undesirable effects following acetylcysteine intravenous perfusion are more likely to appear if the drug is administered too quickly or in an excessive amount. It is therefore recommended to strictly follow the indications reported under “Dosage and Administration”.
Acetylcysteine administration at antidotal dosages may prolong prothrombin time (decrease in prothrombin index, increase in INR). However, it is not established if this is a result of the biologic action of acetylcysteine. In any way, the monitoring of coagulation factors is advisable particularly in cases of liver transplant. Acetylcysteine may interfere with the determination of salicylates (colorimetric method) and with the determination of plasma and urinary ketones (nitroprusside test).
The possible presence of sulfur-like odor does not indicate an alteration of the product but is characteristic of the active ingredient contained in the preparation.
Pregnancy and lactation
There is limited clinical data relating to women exposed to acetylcysteine during pregnancy. Animal studies do not suggest any direct or indirect harmful effects on the pregnancy, embryo/fetal development, birth or postnatal development.
There is no available information on the excretion of acetylcysteine in breast milk.
The product should only be used during pregnancy and lactation after the benefit/risks have been weighed up carefully.
ADVERSE EVENTS
The following adverse reactions were reported during post-marketing experience; their frequency is not known (cannot be estimated from the available data).
System Organ Class
Immune System Disorders
Anaphylactic shock, anaphylactic reaction, anaphylactoid reaction, hypersensitivity
Cardiac Disorders
Tachycardia
Respiratory, thoracic and mediastinal Disorders
Bronchospasm, dyspnea
Gastrointestinal Disorders
Vomiting, nausea
Skin and subcutaneous tissue Disorders
Angioedema, urticaria, flushing, rash, pruritus
General disorders and administration site conditions
Face edema
INVESTIGATIONS
Blood pressure decreased, prothrombin time prolonged
In very rare cases, the occurrence of severe skin reactions such as Stevens-Johnson syndrome and Lyell’s syndrome has been reported in temporal connection with the administration of acetylcysteine.
In most cases, at least one co-suspect drug more probably involved in triggering the reported mucocutaneous syndrome could be identified. Medical advice should be sought immediately if any new changes to the skin or mucous membranes occur, and acetylcysteine should be stopped immediately.
A decrease in platelet aggregation in the presence of acetylcysteine has been confirmed by various investigations. The clinical significance has not been established.
INTERACTIONS
Drug-drug interaction studies have been performed only in adults.
Concurrent administration of nitroglycerin and acetylcysteine has been shown to cause significant hypotension and enhance temporal artery dilation. If concurrent nitroglycerin and acetylcysteine therapy is necessary, patients should be monitored for hypotension, which can be severe, and warned of the possibility of headaches.
Reports of inactivation of antibiotics resulting from acetylcysteine so far only relate to in vitro tests in which the relevant substances were mixed directly. Therefore, dissolution of acetylcysteine formulations concomitantly with other drugs is not recommended.
Acetylcysteine may interfere with colorimetric assay method for salicylate measurement.
Acetylcysteine may interfere with urine ketone test.
OVERDOSAGE
By intravenous route
Symptoms
There is a theoretical risk of hepatic encephalopathy.
Symptoms of overdosage are similar, but more severe than that observed in the case of adverse events.
Treatment
Treatment of overdose is based on immediate discontinuation of the infusion administration and symptomatic treatment and resuscitation. There are no specific antidotal treatments. Acetylcysteine is dialyzable.
STORAGE
Store at temperatures not exceeding 25°C.
Manufactured by
Alfasigma S.p.A.
Via Enrico Fermi, 1
65020 Alanno Pescara, Italy
For Zambon S.p.A.
Via Lillo del Duca 10
20091 Bresso (MI), Italy
Marketing Authorization Holder
The Cathay Drug Co., Inc.
2/F Vernida 1 Condominium
120 Amorsolo St. Legaspi Village, Makati City
Date of Revision: August 2016


Reviews
There are no reviews yet.