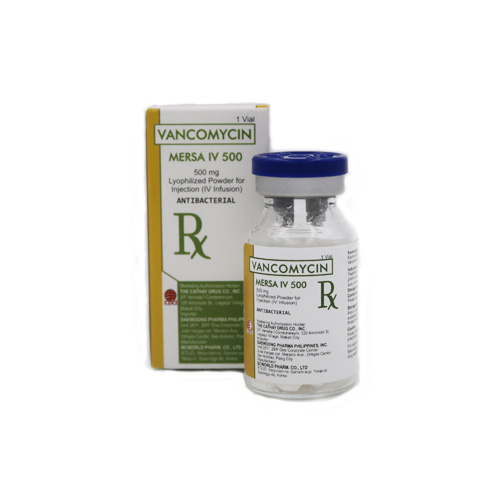
Description
The Cathay Drug Co., Inc.
FORMULATION
MERSA IV 500 500 mg lyophilized Powder (IV Infusion)
Each vial contains:
Vancomycin (as hydrochloride), USP ……………… 500 mg
MERSA IV 1000 1g lyophilized Powder (IV Infusion)
Each vial contains:
Vancomycin (as hydrochloride), USP ……………… 1 g
CLINICAL PHARMACOLOGY
Mechanism of Action
The bactericidal action of vancomycin results primarily from inhibition of cell-wall biosynthesis. Specifically, vancomycin prevents incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits from being incorporated into the peptidoglycan matrix; which forms the major structural component of Gram-positive cell walls. The large hydrophilic molecule is able to form hydrogen bond interactions with the terminal D-alanyl-D-alanine moieties of the NAM/NAG-peptides. Normally this is a five-point interaction. This binding of vancomycin to the D-Ala-D-Ala prevents the incorporation of the NAM/NAG-peptide subunits into the peptidoglycan matrix. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis. There is no cross-resistance between vancomycin and other antibiotics. Vancomycin is not active in vitro against gram-negative bacilli, mycobacteria, or fungi.
PHARMACOKINETICS
Administration and Distribution
In subjects with normal kidney function, multiple intravenous dosing of 1 g of vancomycin (15 mg/kg) infused over 60 minutes produces mean plasma concentrations of approximately 63 mcg/mL immediately after the completion of infusion, mean plasma concentrations of approximately 23 mcg/mL 2 hours after infusion, and mean plasma concentrations of approximately 8 mcg/mL 11 hours after the end of the infusion. Multiple dosing of 500 mg infused over 30 minutes produces mean plasma concentrations of about 49 mcg/mL at the completion of infusion, mean plasma concentrations of about 19 mcg/mL 2 hours after infusion, and mean plasma concentrations of about 10 mcg/mL 6 hours after infusion. The plasma concentrations during multiple dosing are similar to those after a single dose.
Metabolism and Elimination
The mean elimination half-life of vancomycin from plasma is 4 to 6 hours in subjects with normal renal function. In the first 24 hours, about 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration.
Mean plasma clearance is about 0.058 L/kg/h and mean renal clearance is about 0.048 L/kg/h. Renal vancomycin clearance is fairly constant and accounts for 70% to 80% of vancomycin elimination. The volume of distribution ranges from 0.39 to 0.97 L/kg. There are no apparent metabolisms of the drug. Vancomycin is approximately 55% serum protein bound as measured by ultrafiltration at vancomycin serum concentrations of 10 to 100 mcg/mL.
After IV administration of vancomycin, inhibitory concentrations are present in pleural, pericardial, ascetic, atrial appendage tissue and synovial fluids; as well as urine and peritoneal. Vancomycin does not readily diffuse across normal meninges into the spinal fluid; but, when the meninges are inflamed, penetration into the spinal fluid occurs.
INDICATIONS
Treatment of potentially life-threatening infections caused by susceptible gram – positive organisms which cannot be treated by other effective, less toxic antimicrobial drugs, such as the penicillins and cephalosporins.
Severe staphylococcal infections in patients who cannot receive or who have failed to respond to the penicillins or cephalosporins, or who have infections with staphylococci that are resistant to other antimicrobial drugs.
Endocarditis and as prophylaxis against endocarditis in patients at risk from dental or surgical procedures.
Other infections due to staphylococci, including osteomyelitis, pneumonia, septicaemia and soft tissue infections.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
DOSAGE AND ADMINISTRATION
Infusion-related events are related to both the concentration and the rate of administration of Vancomycin (Mersa IV 500 & 1000). Concentrations of no more than 5 mg/mL and rates of no more than 10 mg/min are recommended in adults. In selected patients in need of fluid restriction, a concentration up to 10 mg/mL may be used: use of such higher concentrations may increase the risk of infusion-related events. Infusion-related events may occur, however, at any rate or concentration.
Adults
The usual daily intravenous dose is 500 mg as Vancomycin (Mersa IV 500 & 1000) every 6 hours or 1 g every 12 hours or as prescribed by the physician. Other patient factors, such as age or obesity, may call for modification of the usual intravenous daily dose.
Children
The usual intravenous dosage of vancomycin (Mersa IV 500 & 1000) is 10 mg/kg per dose given every 6 hours. Each dose should be administered over a period of at least 60 minutes. Close monitoring of serum concentrations of vancomycin may be warranted in these patients.
Infants
In both neonates and infants, an initial dose of 15 mg/kg is suggested, followed by 10 mg/kg every 12 hours for neonates in the 1st week of life and every 8 hours thereafter up to the age of 1 month. Each dose should be administered over 60 minutes. Close monitoring of serum concentrations of vancomycin is recommended in these patients.
Dosing recommendations for neonates is depending on post-conception age
*PCA = postconceptual age (gestational age at birth plus chronological age)
*if the serum creatinine concentration is > 1.2 mg/dL, use an initial doseage of 15 mg/kg every 24 hours
*serum creatinine concentration is not used to determine the dosage for this type of patient because of its lack of reliability or because of the lack of information.
Patients with renal function impairment and the elderly
In patients with renal impairment, dosage reduction may be required. In premature infants and the elderly, greater dosage reductions than expected may be necessary because of decreased renal function.
This table is not valid for functionally anephric patients. For such patients, an initial dose of 15 mg/kg of body weight should be given to achieve prompt therapeutic serum concentration. The dose required to maintain stable concentrations is 1.9 mg/kg/24 h. In patients with marked renal impairment, it may be more convenient to give maintenance doses of 250 to 1,000 mg once every several days rather than administering the drug on a daily basis. In anuria, a dose of 1,000 mg every 7 to 10 days has been recommended.
The creatinine clearance is calculated by the following formula
Men:
Creatinine Clearance = Weight (kg) x (140 – age in years)
72 x serum creatinine concentration (mg/dL)
Women : 0.85 x Men’s value
The serum creatinine must represent a steady state of renal function. Such a calculated clearance is an overestimate of actual clearance in patients with conditions: (1) characterized by decreasing renal function, such as shock, severe heart failure, or oliguria; (2) in which a normal relationship between muscle mass and total body weight is not present, such as obese patients or those with liver disease, edema, or ascites; and (3) accompanied by debilitation, malnutrition, or inactivity.
SPECIAL PRECAUTION FOR DISPOSAL AND OTHER HANDLING
For single use. Discard any unused contents.
500 mg – PREPARATION OF THE SOLUTION
At the time of use, add 10 mL of Sterile Water for injection to a 500 mg vial of Vancomycin (Mersa IV 500) Lyophilized Powder for Injection (IV Infusion). Vials reconstituted in this manner will give a solution of 50 mg/mL.
1 g – PREPARATION OF THE SOLUTION
At the time of use, add 20 mL of Sterile Water for injection to a 1 g vial of Vancomycin (Mersa IV 1000) Lyophilized Powder for Injection (IV Infusion).
Further dilution is required depending on the method of administration.
- Intermittent infusion (the preferred method of administration)
- Reconstituted solutions with 10 mL containing 500 mg of vancomycin hydrochloride must be further diluted with at least 100 mL of diluent.
- Reconstituted solutions containing 1 g of vancomycin must be diluted with at least 200 mL of diluent
Sodium Chloride 0.9% Intravenous Infusion or 5% Dextrose Intravenous Infusion are suitable diluents. The desired dose should be administered by intravenous infusion over a period of at least 60 minutes. If administred over a shorter period of time or in higher concentrations, there is a possibility of inducing marked hypotension in addition to thrombophlebitis.
Rapid administration may also produce flushing and a transient rash over the neck and the shoulders.
- Continuous infusion (should only be used if treatment with an intermittent infusion is not possible):
1 to 2 g of Vancomycin (MERSA IV 500 and 1000) in a sufficiently large volume of 5% glucose or 0.9% sodium chloride solution to permit the desired dose to be infused over 24 hours.
CONTRAINDICATIONS
- Patients with history of shock to this drug
- Patients with history of hypersensitivity to this drug, peptide antibiotics or aminoglycoside antibiotics
WARNINGS
- To prevent the emergence of resistant microorganisms by the therapy with this drug, it is desirable to check susceptibility and to administer for the shortest period as possible
- Due to the possibility of developing toxic effects in the presence of the continuously maintained serum concentration and prolonged high blood concentrations, this drug should be used with caution in patients with renal failure with the dosage adjustment. In the treatment of patients with the history of renal disorder and in those who are receiving concomitant treatment with aminoglycosides, serial tests of renal function must be performed and the appropriate dose regimens adhered to in order to reduce the risk of nephrotoxicity to a minimum.
- In case of repeated oral administration for the treatment of pseudomembranous colitis by Clostridium difficile, if the sign of improvement of symptoms such as diarrhea, abdominal pain and fever does not appear clearly within 7 – 10 days, treatment with this drug should be discontinued
- Prolonged use of this drug may result in the overgrowth of non-susceptible microorganisms. If superinfection occurs during therapy, this drug should be discontinued and appropriate measures should be taken.
- Serial tests of autditory function is advisable in order to minimize the risk of ototoxicity.
- During therapy with this drug, serum test, urine analysis and tests of liver function and renal function should be conducted periodically. Patients who will undergo prolonged therapy with this drug or those who are receiving concomitant drugs that may cause neutropenia should have periodic monitoring of the leukocyte count.
PRECAUTIONS
- As a rule, this drug should not be administered to patients who have hearing loss by peptide antibiotics or aminoglycoside antibiotics or other underlying hearing loss, but if absolutely necessary, this drug should be used with caution.
- Patients with renal impairment such as acute urinary retention (because this drug is accumulated due to delayed excretion, it should be administered cautiously with the monitoring of serum concentration)
- Patients with hepatic impairment (hepatic impairment may be caused or deteriorated)
- The elderly
- Premature infants, neonates
- Patients with impaired vestibular organ or impaired cochlea
PRECAUTIONS IN ADMINISTRATION
- This drug is irritating to tissue, and it must not be given by intramuscular injection. Careless intravenous administration extravasation may cause pain, tenderness on pressure, necrosis, etc.
- Thrombophlebitis may occur by intravenous administration with high concentration, but the frequency and severity of which can e minimized by enough dilution (2.5 – 5 g/L), slow infusion and rotation of venous access sites.
- Rapid bolus administration over several minutes may cause hypotension and rarely, cardiac arrest. To avoid rapid-infusion-related reactions, this drug should be administered over a period of not less than 60 minutes. Stopping the infusion usually results in prompt cessation of these reactions.
- The safety and efficacy of administration via lumbar vertebrae and cerebral ventricles are not yet evaluated yet.
- Due to the low pH of solution of this drug, mixing with other substances may cause physical or chemical instability. Do not mix this drug with alkaline solutions.
- Solution of this drug and beta-lactam antibiotics are physically incompatible. The likelihood of precipitation increases with higher concentrations of this drug. It is recommended to adequately flush the intravenous lines between administrations of these antibiotics. It is also recommended to dilute solutions of this drug to 5 mg/mL or less.
- Administration of this drug into the vitreous body is not permitted: a precipitation has been reported when this drug and ceftazidime were injection into the vitreous body of patients with endophthalmitis using separate syringe and needle. Precipitate gradually dissolved, and two months later, vitreous cavity became transparent completely and sight was improved.
- Before injection, this drug should be inspected visually for particulate matter and discoloration.
USE IN PREGNANT WOMEN AND NURSING MOTHERS
- In reproduction studies on rats at doses up to 5 times the human dose and rabbits at doses up to 3 times the human dose, fetotoxicity has not been reported.
- In a controlled clinical study, the potential ototoxic and nephrotoxic effects of vancomycin on infants were evaluated when the drug was administered to pregnant women for serious staphylococcal infections complicating intravenous drug abuse. Vancomycin was found in cord blood. No sensorial hearing loss or nephrotoxicity attributable to vancomycin was noted.
- One infant whose mother received vancomycin in the third trimester experienced conductive hearing loss that was not attributed to the administration of vancomycin. Because the number of patients treated in this study was limited and vancomycin was administered only in the second and third trimesters, it is not known whether vancomycin causes fetal harm.
- Because the safety of this drug during pregnancy has not been established, this drug should be administered to pregnant women or women of child bearing potential only if therapeutic benefit is judged to outweigh the potential risk. If administered, serum concentration should be carefully monitored to reduce fetotoxicity.
- Because this drug is excreted in human milk, nursing mothers should avoid administration of this drug. If treatment is unavoidable, nursing should be discontinued.
PEDIATRIC USE
Premature infants, neonates and toddlers are at the stage of renal development. Because high serum concentration may last for a long time due to prolonged half-life of this drug in these groups, caution should be taken in the administration.
GERIATRIC USE
Due to the age-related reduction in renal function, renal function should be tested before and during administration, and this drug should be administered with caution by reducing doses according to the decreased renal function.
DRUG INTERACTIONS
- The frequency of anaphylactoid reactions including flushing, histamine like response, hypotension, wheezing, dyspnea, urticarial, and pruritus may increase with the concomitant administration of anesthetic agents, it can be minimized by the administration of this drug as a 60-minute infusion prior to anesthetic induction.
- Concurrent and/or sequential systemic or topical use of other potentially neurotoxic and/or nephrotoxic drugs such as amphotericin B, aminoglycosides, bacitracin, polymixin B, colistin, viomycin, or platinum antitumor drugs (cisplatin, nedaplatin, etc.) may increase adverse reactions, so they should be administered with caution.
- Change of international normalized ratio (INR): In patients taking anticoagulant with antibiotics including this drug concomitantly, increased anticoagulant activity has been reported. Infectious disease (accompanied with inflammatory process) and age and general condition of patients are risk factors. Although interaction of this drug with warfarin has not been established through clinical trials, INR monitoring should be performed, and if necessary, the dosage of oral anticoagulant should be properly adjusted. Some kinds of antibiotics, especially fluoroquinolones, macrolides, cyclins, co-trimoxazole and some cephalosporines, showed severe cases.
ADVERSE EFFECTS
- Neuropsychological system: because shock and anaphylactoid symptom may rarely occur, patients should be monitored closely. If any adverse reaction is observed, administration should be discontinued and appropriate measures should be taken
- Digestive system: loose feces, diarrhea, nausea, vomiting and abdominal pain may rarely occur
- Blood system: oligocythemia, leukopenia, thrombocytopenia and eosinophilia may rarely occur. Reversible neutropenia has been reported and neutropenia appears to be promptly reversible when this drug is discontinued. Although a casual relationship has not been established, reversible agranulocytosis (granulocytes <500/mm3) has been reported rarely.
- Central nervous system: because the eighth cranial nerve impairment such as dizziness, tinnitus, hearing loss, etc. may occur, dose monitoring is required including testing hearing activity. As a rule, this drug should not be administered to patients with these symptoms, but if absolutely necessary, this drug should be used with caution. Hearing loss associated with this drug has been reported in patients who had kidney dysfunction or a pre-existing hearing loss or were receiving concomitant treatment with an ototoxic drug.
- Liver: Because increased bilirubin, AST, ALT and ALP, rarely, increased LDH, y-GTP and LAP may occur, patients should be observed closely including regular monitoring. If any adverse reaction occurs, appropriate measure should be taken including discontinuation of administration.
- Kidney: because renal impairment such as increased BUN and creatinine may occur, patients should be observed closely including regular monitoring. As a rule, this drug should not be administered to patients with these adverse reactions, but if absolutely necessary, this drug should be used with caution. Interstitial nephritis have been reported rarely in patients who were given aminoglycoside antibiotics or who had pre-existing kidney dysfunction. When this drug was discontinued, azotemia resolved.
- Skin: Exfoliative dermatitis, bullous dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis may occur.
- Hypersensitivity reactions: When rash, erythema, flushing, hypotension, wheezing, dyspnea, urticarial and pruritus occurs, appropriate measures should be taken. Rapid infusion may also cause red-man’s syndrome (erythema bleeding of face, neck and upper body), pain of chest and abdomen and, muscle spasm. These reactions usually resolve within 20 minutes but may persist for several hours. In animal studies where overdose of this drug was administered rapidly with high concentration, hypotension and bradycardia occurred. These adverse reactions rarely occur when this drug is slowly administered over a period of not less than 60 minutes, and there was no injection-related adverse reactions when this drug was administered to healthy human with a rate of not more than 10 mg/minute.
- Others: occasionally fever, angialgia, phlebitis, rarely, nausea, chills and vasculitis may occur, pseudomembranous colitis by Clostridium difficile also has been reportedin patients who had administered this drug intravenously.
OVERDOSAGE
Symptoms: Renal disorders such as acute renal failure and eighth cranial nerve impairment such as hearing loss may occur.
Measures: Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to result in increased vancomycin clearance. The medial lethal intravenous dose is 319 mg/kg in rats and 400 mg/kg in mice.
CAUTION
Foods, Drugs, Devices and Cosmetics Act prohibits disoensing without prescription.
STORAGE
Prior to reconstitution, vials should be stored at temperatures not exceeding 30°C.
Reconstituted solution should be stored between 2-8°C. Protect from light.
PRODUCT DESCRIPTION
Vancomycin (Mersa IV 500) is a white or almost white free-flowing lyophilized powder, supplied in a USP Type I clear glass vial with rubber stopper, sealed with tear off aluminium seal and blue flip-off plastic cap.
Vancomycin (Mersa IV 1000) is a white or almost white free-flowing lyophilized powder, supplied in a USP Type I clear glass vial with rubber stopper, sealed with tear off aluminium seal and gray flip-off plastic cap.
Reconstituted Solution: white or white to pale yellowish solution.
AVAILABILITY
- Vancomycin (MERSA IV 500) 500 mg Lyophilized Powder for Injection (IV Infusion): USP Type I Clear Glass Vial (Box of 1’s)
- Vancomycin (MERSA IV 1000) 1 g Lyophilized Powder for Injection (IV Infusion): USP Type I Clear Glass Vial (Box of 1’s)
Manufacturer
BC WORLD PHARM. CO., LTD.
872-23, Yeojunam-ro, Ganam-eup, Yeoju-si
Gyeonggi-do, Korea
Marketing Authorization Holder
THE CATHAY DRUG CO., INC.
2/F Vernida 1 Condominium
120 Amorsolo St., Legaspi Village, Makati City
Date of Revision: January 2018

Reviews
There are no reviews yet.